Background
Chimeric antigen receptor T-cell therapies (CAR-T) significantly improve survival and health-related quality of life (HRQoL) in clinical trials of relapsed hematologic malignancies. However, the impact of CAR-T on real-world longitudinal patient-reported outcomes (PROs), particularly in the immediate post-CAR-T period during which patients are hospitalized and at risk for acute CAR-T-related toxicities, has not been studied. Further, few CAR-T studies have utilized Patient-Reported Outcomes Measurement Information System (PROMIS) survey instruments, which are recommended to enable comparison of PROs across various CAR-T products and disease types. To characterize real-world HRQoL and symptom burden in patients receiving CAR-T, we integrated PROMIS PRO instruments into a prospective cellular therapy registry.
Methods
The University of Chicago Cellular Therapy Biobank prospectively administers the PROMIS v1.2 Global Health and PROMIS-29 Profile v2.1 instruments to CAR-T recipients. PRO instruments are administered at the following timepoints, anchored to the day (D) of CAR-T receipt: baseline (at time of apheresis), D+1, D+15, D+28, D+90, D+180, and then every 3 months until the 2-year timepoint. The Biobank was queried for all patients who completed a baseline and at least one post-CAR-T survey. PROMIS T-scores were analyzed by one-way repeated measures ANOVA up to the 12-month timepoint. PROMIS T-scores were longitudinally visualized as mean change from baseline. Minimally important differences (MID) that were clinically significant were defined as T-score changes of at least 2. Statistical analyses were performed in STATA, version 17.0.
Results
There were 68 patients available for PRO analysis. Median age was 65 years (range, 24-80) and 63% were male. Most patients had large B-cell lymphomas (n = 27, 40%) or multiple myeloma (n = 27, 40%). Median prior lines of therapy were 4 (range, 1-12), with only 3 patients receiving 1 prior therapy. The majority of patients received CD19-targeted products (n = 35, 51%), followed by BCMA-targeted products (n = 27, 40%). Patients lived a median of 36 miles from the CAR-T center (range, 2-544), with 65% living over 30 miles away. Survey completion rates at timepoints were as follows: 73% at D+15, 82% at D+28, 84% at D+90, 76% at D+180, and 95% at 12 months.
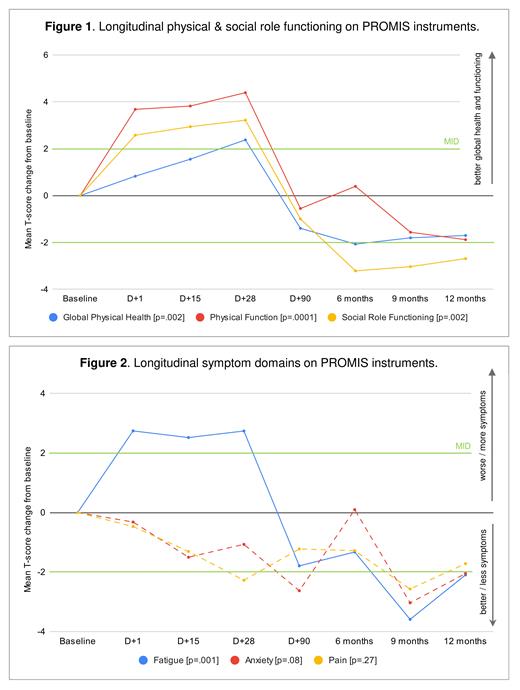
For PROMIS Global Health, there was a significant longitudinal change in Global Physical Health (p = .002), with a clinically meaningful improvement by D+28 which was no longer observed by D+90 ( Figure 1), but no significant change in Global Mental Health (p = .09). For PROMIS-29 Profile, there were significant longitudinal changes in physical function (p = .0001), fatigue (p = .001), and social role functioning (p = .002), but not anxiety (p = .08), depression (p = .45), sleep (p = .92), or pain (p = .27). There were clinically meaningful improvements in both physical and social role functioning in the first 28 days after CAR-T which were no longer observed by D+90 ( Figure 1). Fatigue significantly worsened in the first 28 days after CAR-T to a clinically meaningful degree, but subsequently improved to better than baseline after D+90 ( Figure 2).
With a median follow-up of 9 months (range, 1-28), 40% of patients had post-CAR-T disease progression. Relapses occurred a median of 4.9 months after CAR-T, with 31% of relapses (n = 8) occurring before D+90. There were 10 deaths (15%) at a median of 6.7 months after CAR-T.
Conclusions
In this real-world prospective PRO analysis, there were clinically meaningful improvements in several HRQoL domains in the first month after CAR-T, namely physical and social functioning, despite significantly worsening fatigue during the same time period. However, these HRQoL improvements diminished after D+28, concurrent with the transition of patients from the CAR-T center to home. These results suggest that intensive support by the inpatient and outpatient care teams may improve physical HRQoL during the immediate post-CAR-T period. In addition, given the low rate of relapses before D+90 in this cohort, worsening patient-reported functioning after D+28 may be driven by changes in care support after discharge to home. Interventions to optimize the post-CAR-T transition to home are warranted, as well as studies utilizing PROMIS PROs to identify patients with deteriorating HRQoL who may benefit from augmented support and/or closer surveillance for relapsed disease.
Disclosures
Kline:MorphoSys: Consultancy; Kite/Gilead: Consultancy; Karyopharm: Consultancy; Verastem: Consultancy, Research Funding; Merck: Consultancy, Research Funding; iTeos, Secura Bio: Research Funding; Seagen: Consultancy. Bishop:Incyte: Honoraria, Other: Travel support, Speakers Bureau; ADC Therapeutics: Speakers Bureau; Chimeric Therapeutics: Consultancy; Tmunity: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Sana Biotechnology: Consultancy; Triumvira: Research Funding; Arcellx: Consultancy, Research Funding; WindMIL Therapeutics: Consultancy; Bluebird Bio: Consultancy; Autolus: Consultancy, Research Funding; Iovance: Consultancy; Servier: Speakers Bureau; Immatics: Research Funding; KITE/Gilead, Novartis, CRISPR Therapeutics, Autolus Therapeutics, BMS/JUNO Therapeutics, Incyte, Sana Biotechnology, Iovance Biotherapeutics, In8bio, Chimeric Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS, Kite/Gilead, Servier, AstraZeneca, ADC Therapeutics, Incyte: Speakers Bureau; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; BMS: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support, Research Funding; Sanofi: Honoraria, Speakers Bureau; Celgene: Honoraria. Cook:Academic Impressions, Inc.: Consultancy. Riedell:Karyopharm Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; MorphoSys: Research Funding; Nektar Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy; Tessa Therapeutics: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Xencor: Research Funding; CRISPR Therapeutics: Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy; Nkarta: Research Funding; Roche: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Calibr: Research Funding; CVS Caremark: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal